by Uwe Blesching on January 24, 2021
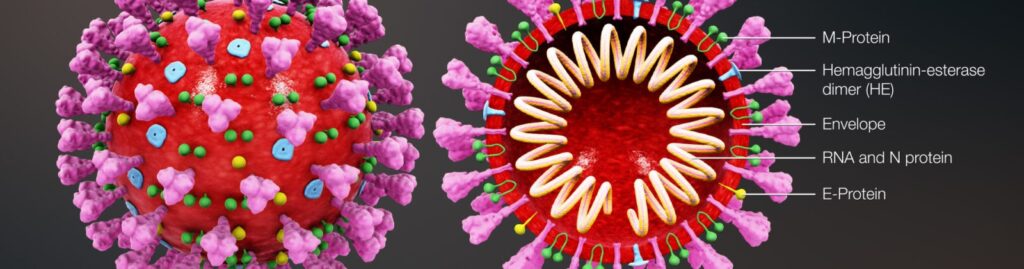
When looking at a picture taken by a transmission electron micrograph able to visualize nano-dimensional objects such as a corona virus for example you can see a crown-like near spherical envelope with protruding spikes similar to the strange ring of light you can observe when looking at the corona of the sun. As such it is perhaps easier to understand how the virus got its name from the Latin word corona meaning crown.
Coronaviruses are a family of hundreds of viruses that typically cause symptoms not unlike the common cold or flu. Until very recently and unlike the cold or flu corona viral infections affected only a relatively small numbers of people.
All of that changed in the year 2002 with the emergence of first epidemic of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in southern China. By the time this epidemic was done the CDC reports ~8,000 patients had been infected in total with a ~10% mortality rate (no new cases since 2004).11.
Humanities next brush with a member of the corona viral family came ten years later. This time originating in Saudi Arabia (2012) causing Middle East Respiratory Syndrome (MERS-CoV) with approximately 2,500 confirmed cases and a fatal rate of ~35% (MERS-CoV is still active).2.
Fast forwarding to the next outbreak brings us to 2019 when news of a novel and mysterious respiratory illness reaches us from Wuhan, China. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) is the strain of coronavirus that caused coronavirus disease 2019 (COVID-19).
To the surprise and shock the global population this outbreak grew rapidly from an epidemic to a global pandemic. By January 21, 2021 the global count of corona virus cases was estimated to have surpassed 97 million people with an estimated 2.1 million fatalities (~2.2%) (for live update go to link in endnotes.)3.
Corona viruses can be transmitted by zoonotic means that is they can be transmitted between animals and humans. The vectors for transmission are posited to be bats (considered primary viral reservoirs), camel (or dromedary), with other animals (e.g. masked palm civets ) acting as possible intermediaries.
Patients who contract COVID-19 do not all get sick in the same way or with the same intensity of symptoms, which range from mild, moderate, or severe to fatal, depending on the resilience or constitution of the affected person.
The CDC (Centers for Disease Control and Prevention) suggest that—based on the, better-understood member of the corona viral family, i.e., the 2012 appearance of the Middle East Respiratory Syndrome-Related Coronavirus, MERS-CoV—symptoms are likely to appear between 2 and 14 days after exposure (typically via airborne droplets).4.
This estimate was fine-tuned by the most recent study from Johns Hopkins Bloomberg School of Public Health (2020) which discovered that the median incubation period for COVID-19 of was approximately 5 days5.
The Organização Mundial da Saúde (OMS) lists as most common symptoms (in descending order): fever, weakness (tiredness, fatigue), dry cough. The WHO also lists less commonly reported symptoms such as shortness of breath, aches and pains (i.e., bone, joint, muscle pains, headaches), sore throat, with a minority of patients reporting nasal congestion, nausea, and diarrhea6.
Some people who have tested positive for corona virus have not shown any or only very mild symptoms; and while earlier estimates suggested that four out of five patients will recover without developing complications a more recent meta-analyses (September 2020) including a total of 94 COVID trials found that only one out of five infected people will remain asymptomatic7.
People at higher risk of developing serious complications include those with an impaired immune system due to other medical conditions such as diabetes, chronic obstructive pulmonary disorder (COPD), or cancer patients receiving chemotherapies, for example.
Serious developments that warrant immediate medical attention include acute respiratory distress syndrome (ARDS) which is causes by cytokine storms aka cytokine storm syndrome (CSS).
At this stage of the disease a vast number of virally infected cells have been killed by the body’s immune responses and it is the sheer abundance of these dead cells that are significantly contributing to generate the symptoms such as signs of shock (inadequate tissue perfusion) such as rapid and labored breathing, pale, blueish discolored lips and skin, confusion, hypotension (low blood pressure), rapid heart rate, and you may see the appearance of co-morbidities such as sepsis, pneumonia, pleuritic pain, aspiration (phlegm from the upper airways enters the lung), and organ failure, which if not reversed can cause death.
ARDS requires 911/ER/ICU intervention such as intubation (a plastic tube is placed into the trachea) with an attachment to a manual (a person) or automatic ventilator, intravenous fluids, and appropriate pharmaceutical interventions to counteract poor oxygenation, hypotension and other clinical manifestations.
To-date allopathic medicine offers no proven treatment or cure. However, vaccine development to control of viral replication has been completed and roll-out has begun.
Future focus will be to make sure that the vaccines do not trigger serious adverse effects such as hyperactivation of immune responses. At the same time novel anti-inflammatory therapies to address cytokine storm syndrome will still need to be developed.
A group of researchers from numerous US-based academic institutions (2020)(+) conducted a review and subsequently posit that four unconventional but readily available immunomodulatory agents are ready for clinical trials to determine their efficacy in the treatment of Corona viral disease: low-dose oral interferon alpha, microdose DNA, low-dose thimerosal, and phytocannabinoids (e.g. CBD, THC).8
Cannabis and Corona Viral Diseases: A Mini Review
This review contains 18 pre-clinical trials all published in 2020 and 2021 that directly examined components of the endocannabinoid system and/or cannabinoid-based therapeutics in the treatment context of corona viral diseases. More specifically, 4 lab-test, 2 animal experiment and 12 reviews or meta-analyses are beginning to offer a better understanding and insight.
Laboratory trials: A group of researchers from Canada (2020)(+) discovered that based on the hypothesis that modulation of angiotensin-converting enzyme 2 (ACE2) levels in viral gateway tissues (i.e. mucus membranes, lungs) may be an effective strategy for decreasing susceptibility to COVID-19.
This team developed over 800 new cannabis sativa cultivars in the hope that high-CBD cannabis extracts may be used to down-regulate ACE2 expression in affected tissue sites. The trial identifies 13 high-CBD cannabis sativa extracts that decrease ACE2 protein levels.9.
Building on the previous insights the same team from Canada (2020)(+) used an artificial human 3-D model of various tissues typically affected by the virus (e.g. lung, oral and nasal mucosa for example) to further test the 13 CBD abundant extracts of cannabis that were found to down-regulate ACE2 gene expression and ACE2 protein levels (both potential markers involved in determining viral entry).
The effective chemotype II and III sub-ratios of the 13 CBD-rich cannabis extracts ranged include the following (THC:CBD) ratios: 1:1.5, 1:3, 1:9, 1:19, 1:20, 1:21 (x2), 1:24, 1:27, 1:28, 1:34, 1:35, and 1:54. The authors posit that select high-CBD cannabis extracts may play a novel role when developing potential prevention regimens10.
Based on the recent finding that implicate 3C-like protease (3CLpro), a key enzyme for coronavirus replication, these researchers from India (2020(+) wanted to test the effects of the terpene eucalyptol on 3CLpro. Results showed effective binding of eucalyptol to COVID-19 proteinase. As such the authors posit that eucalyptol may potentially represent a novel target mechanism to inhibit COVID-19 replication11.
Utilizing a human 3D skin artificial EpiDermFTTM tissue model a group of scientists from Canada (2021)(0) tested the effects of 7 cannabis sativa extract containing various amounts of cannabinoids (THC, CBD, CBGA, CBN) as well as different terpene profiles (e.g. β-caryophyllene, linalool) on two major pro-inflammatory players in cytokine storm pathogenesis i.e. TNFα and IL-6.
Resulting data revealed that out of 7 extracts tested 3 were most effective (2 were effective) 1 extracts had no effect and 1 other produced a negative effect in the down-regulating of pro-inflammatory genes coding for TNFα and IL-6.
Extracts #4, #8 and #14 were most effective. Extract #12 produced a negative effect. Composition of each compound:
- Extract #4 contained a total of 33.6%THC, 1.72D, 0.32GA, 0.14N
- Extract #8 contained a total of 32.5%THC, 0.33D, 0.49GA, 0.05N (dominant in β-caryophyllene)
- Extract #14 contained a total of 44.3%THC, 1.1D, 0.23GA, 0.32N
- Extract #12 contained a total of 43.2%THC, 1.8D, 0.92GA, 0.12N (dominant in linalool and guaiol).12
Animal Experiments: A US-based group (2020)(+) used a mouse model of acute respiratory distress syndrome (ARDS). When left untreated the mortality rate of the test animals was 100%.
In direct contrast, the administration of Δ9-tetrahydrocannabinol (THC) led to a 100% survival rate, decreased lung inflammation, and suppressed of cytokine storm syndrome. The authors write: “Collectively, this study suggests that the activation of cannabinoid receptors may serve as a therapeutic modality to treat ARDS associated with COVID-19.”13.
Researchers from Belgium and the US (2020(+) discovered that the administration of CBD downregulated proinflammatory cytokines and reduced clinical symptoms of Poly I:C-induced acute respiratory distress syndrome (ARDS) in mice.
The authors conclude that CBD may play protective role during ARDS i.e. reducing the cytokine storm, protecting pulmonary tissues, and re-establishing inflammatory homeostasis.14.
Meta-analyses, Reviews: This team of researchers from the University of South Carolina (2020)(0) proposes that targeting cannabinoid receptor sites deserves intense investigation as a novel approach to treat COVID-1915.
The authors of this review from the United Arab Emirates and the US (2020)(0) posit that CB2 agonism, due to immunomodulatory, anti-inflammatory, and antiviral properties may show activity against COVID-1916.
Canadian researcher (2020)(+) suggests that since CBD is already widely used in the clinical setting and has a favorable safety profile, the results of additional, in vitro and in vivo trials or proof-of-concept studies could provide the needed evidence required before embarking on costly and labor-intensive clinical trials.17.
A team from Iran (2020)(0) proposes that opioids and/or cannabinoids may serve as a potential therapeutic approach in the treatment of COVID-19 patients18.
A US/Israeli-based researcher (2020)(0) finds that CBD is a reasonable candidate to be studied in the pre-clinical setting to determine its potential efficacy19.
South Africa and UK-based scientists (2020(0) looking for novel treatment strategies in the treatment or prevention of COVID-19 examined a number of herbs including cannabis and call for clinical trials to evaluate their treatment potentials20.
This US-based team of reviewer (2020(-) concluded that heavy, problematic cannabis use may increase chances of hospitalization due to COVID-19 respiratory complications.21
Another US-based analysis (2020(+) posits that cannabinoid-based therapeutics may be of use in attenuating SARS-CoV-2 infections by quelling the cytokine storm. However, they acknowledge that clinical trials are needed22.
A group of Chinese and Italian scientists (2020)(+) present their hypothesis that CBD has the potential to limit severity and progression of COID-19 based on: Cannabis extracts down-regulate the expression of the two key receptors for SARS-CoV2 in several models of human epithelia;
- CBD induces a number of immunomodulatory and anti-inflammatory effects;
- CBD can attenuate cytokine storm syndrome;
- CBD display antiviral activity via PPARγ agonism can inhibit the genesis of pulmonary fibrosis23.
Spanish and Portuguese researchers (2020)(-) concluded that people who smoke or vape cannabis are at greater risk of contracting COVID-1924.
An Italian team (2020)(+) hypothesizes that CB2 receptor can be a therapeutic target in COVID-19 pandemic emergencies25.
Summary- Chemotype- Terpene-Specific Considerations
At the time of this writing there are no clinical trials (but they are on their way) providing us with specific evidence-based information to make more practical or discerning decisions about the use of cannabinoid-based therapeutics in this novel pandemic.
So, any claims of cannabis being a cure or a detriment in the treatment context of patients with COVID-19 are presently unsubstantiated by clinical trials.
In summary, the most studied cannabis constituent in the context of corona viral diseases is CBD, followed by THC and then by the terpene eucalyptol. CB2 agonism has been proposed as a possible target receptor. All cannabis chemotypes I, II, and III have been utilized in various pre-clinical trials reviewed in this section and as such may be of value.
Notes:
(+) Key findings suggest therapeutic effects.
(0) Key findings demonstrated mixed or inconclusive results.
(-) Key findings suggest detrimental effects.
References
1. Center for Disease Control and Prevention (CDC). Severe Acute Respiratory Syndrome (SARS). Basic Fact Sheet. https://www.cdc.gov/sars/about/fs-sars.html
2. Cristiano Salata, Arianna Calistri, Cristina Parolin, Giorgio Palù, Coronaviruses: a paradigm of new emerging zoonotic diseases, Pathogens and Disease, Volume 77, Issue 9, December 2019.
3. John Hopkins University and Medicine. Coronavirus Resource Center. https://coronavirus.jhu.edu/map.html
4. Centers for Disease Control and Prevention (CDC). Corona Virus Disease 2019 (COVID-19). Symptoms & Testing. https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html
5. Lauer SA, Grantz KH, Bi Q, Jones FK, Zheng Q, Meredith HR, Azman AS, Reich NG, Lessler J. The Incubation Period of Coronavirus Disease 2019 (COVID-19) From Publicly Reported Confirmed Cases: Estimation and Application. Ann Intern Med. 2020 May 5;172(9):577-582.
6. World Health Organization (WHO). Coronavirus; Symptoms. https://www.who.int/health-topics/coronavirus#tab=tab_3
7. Buitrago-Garcia D, Egli-Gany D, Counotte MJ, Hossmann S, Imeri H, Ipekci AM, Salanti G, Low N. Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: A living systematic review and meta-analysis. PLoS Med. 2020 Sep 22;17(9):e1003346.
8. Mamber SW, Krakowka S, Osborn J, Saberski L, Rhodes RG, Dahlberg AE, Pond-Tor S, Fitzgerald K, Wright N, Beseme S, McMichael J. Can Unconventional Immunomodulatory Agents Help Alleviate COVID-19 Symptoms and Severity? mSphere. 2020 May 13;5(3):e00288-20.
9. Wang B, Kovalchuk A, Li D, Rodriguez-Juarez R, Ilnytskyy Y, Kovalchuk I, Kovalchuk O. In search of preventive strategies: novel high-CBD Cannabis sativa extracts modulate ACE2 expression in COVID-19 gateway tissues. Aging (Albany NY). 2020 Nov 22;12.
10. Wang, B.; Kovalchuk, A.; Li, D.; Ilnytskyy, Y.; Kovalchuk, I.; Kovalchuk, O. In Search of Preventative Strategies: Novel Anti-Inflammatory High-CBD Cannabis Sativa Extracts Modulate ACE2 Expression in COVID-19 Gateway Tissues. Preprints 2020, 2020040315.
11. Sharma, A.D.; kaur, I. Eucalyptol (1,8 cineole) from Eucalyptus Essential Oil a Potential Inhibitor of COVID 19 Corona Virus Infection by Molecular Docking Studies . Preprints 2020, 2020030455.
12. Kovalchuk A, Wang B, Li D, Rodriguez-Juarez R, Ilnytskyy S, Kovalchuk I, Kovalchuk O, . Fighting the storm: could novel anti-TNFα and anti-IL-6 C. sativa cultivars tame cytokine storm in COVID-19?. Aging (Albany NY). 2021. https://doi.org/10.18632/aging.202500
13. Mohammed A, F K Alghetaa H, Miranda K, et al. Δ9-Tetrahydrocannabinol Prevents Mortality from Acute Respiratory Distress Syndrome through the Induction of Apoptosis in Immune Cells, Leading to Cytokine Storm Suppression. Int J Mol Sci. 2020;21(17):6244.
14. Khodadadi H, Salles ÉL, Jarrahi A, Chibane F, Costigliola V, Yu JC, Vaibhav K, Hess DC, Dhandapani KM, Baban B. Cannabidiol Modulates Cytokine Storm in Acute Respiratory Distress Syndrome Induced by Simulated Viral Infection Using Synthetic RNA. Cannabis Cannabinoid Res. 2020 Sep 2;5(3):197-201.
15. Nagarkatti P, Miranda K, Nagarkatti M. Use of Cannabinoids to Treat Acute Respiratory Distress Syndrome and Cytokine Storm Associated with Coronavirus Disease-2019. Front Pharmacol. 2020;11:589438.
16. Nagoor Meeran MF, Sharma C, Goyal SN, Kumar S, Ojha S. CB2 receptor-selective agonists as candidates for targeting infection, inflammation, and immunity in SARS-CoV-2 infections. Drug Dev Res. 2020 Nov 15.
17. Costiniuk CT, Jenabian MA. Acute inflammation and pathogenesis of SARS-CoV-2 infection: Cannabidiol as a potential anti-inflammatory treatment?. Cytokine Growth Factor Rev. 2020;53:63-65.
18. Tahamtan A, Tavakoli-Yaraki M, Salimi V. Opioids/cannabinoids as a potential therapeutic approach in COVID-19 patients. Expert Rev Respir Med. 2020;14(10):965-967.
19. Kevin P. Hill.Cannabis and Cannabinoid Research.Jun 2020.118-120.
20. Dzobo K, Chiririwa H, Dandara C, Dzobo W. Coronavirus Disease-2019 Treatment Strategies Targeting Interleukin-6 Signaling and Herbal Medicine. OMICS. 2020 Aug 26.
21. Hatoum AS, Morrison CL, Winiger EA, Johnson EC, Agrawal A, Bogdan R. Genetic Liability to Cannabis Use Disorder and COVID-19 Hospitalization. medRxiv [Preprint]. 2020 Nov 18:2020.11.15.20229971.
22. Onaivi ES, Sharma V. Cannabis for COVID-19: can cannabinoids quell the cytokine storm? Future Sci OA. 2020 Aug 13;6(8):FSO625.
23. Esposito G, Pesce M, Seguella L, Sanseverino W, Lu J, Corpetti C, Sarnelli G. The potential of cannabidiol in the COVID-19 pandemic. Br J Pharmacol. 2020 Nov;177(21):4967-4970.
24. Pascual Pastor F, Isorna Folgar M, Carvalho N, Carvalho F, Arias Horcajadas F. Therapeutic Cannabis and COVID-19: between opportunism and infoxication. Adicciones. 2020 Jul 1;32(3):167-172.
25. Rossi F, Tortora C, Argenziano M, Di Paola A, Punzo F. Cannabinoid Receptor Type 2: A Possible Target in SARS-CoV-2 (CoV-19) Infection? Int J Mol Sci. 2020 May 27;21(11):3809.
